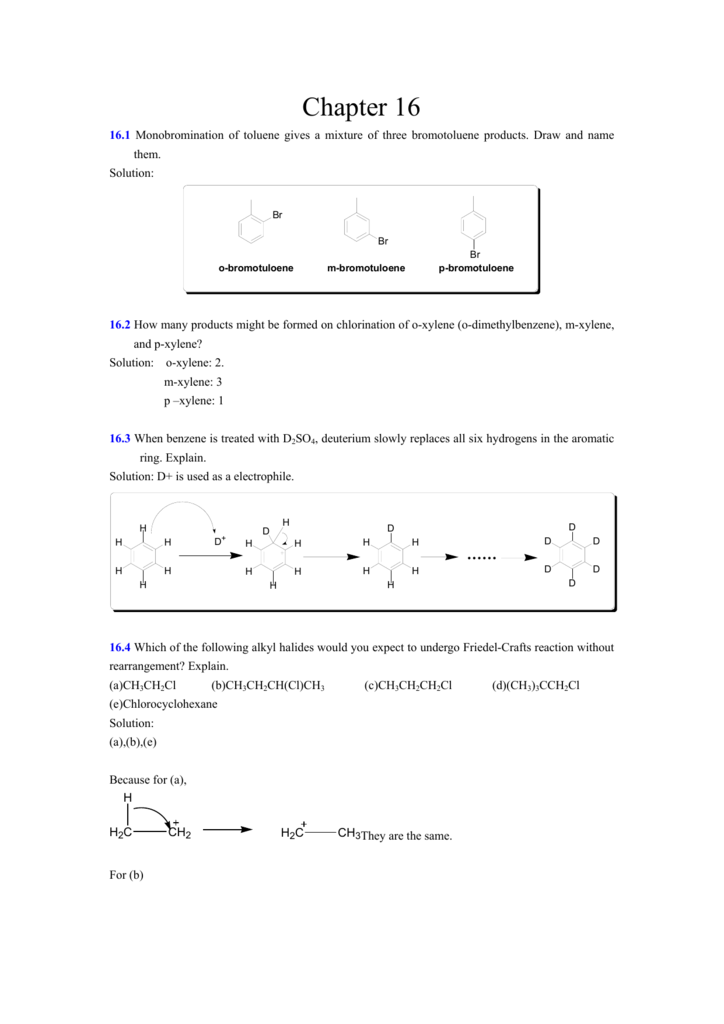
[最も共有された! √] p-xylene bromination 586331-P-xylene bromination
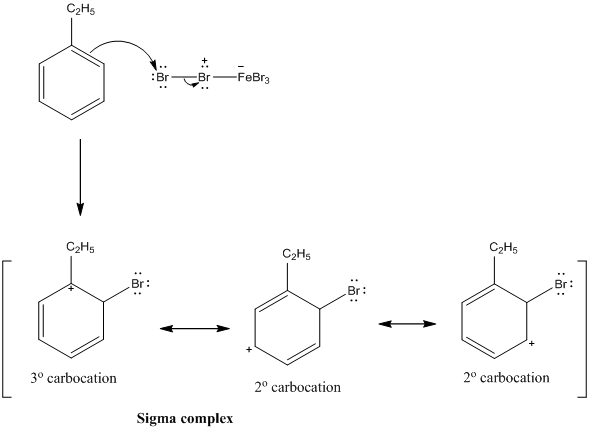
Ch17 Reactions of Aromatic Compounds (landscape)docx Page4 Bromide ion from the FeBr 4can act as a weak base to remove the proton, thus generating the aromatic product, HBr, and regenerating the catalyst (FeBr 3) The formation of the sigma complex is an endothermic and energetically unfavorable process it is therefore theA new process for the generation of paraxylene (pxylene) through benzene alkylation with CH 3 Br has been proposed for the first time CH 3 Br is prepared by the catalytic bromination of methane (the main component of natural gas), and benzene can be prepared from methane dehydroaromatization Over the optimized P 2 O 5 –ZnO/HZSM5 catalyst, benzene conversion is 436% and pxyleneBromination of the substituted toluenes in the presence of ammonium cerium(IV) sulfate and inmore The mono and polybromination of benzene, halogenobenzenes, toluene, pxylene, anisole, biphenyl, benzotrifluoride, benzoic acid, pnitro and pcarboxytoluene, pmethoxybenzonitrile, tetralin, and naphthalene were studied in trifluoroacetic
Answered Br2 Br R Hulene Br Not Formed Bartleby
P-xylene bromination
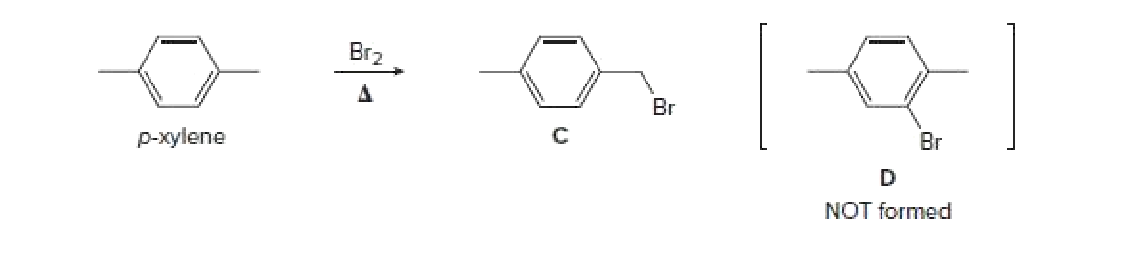
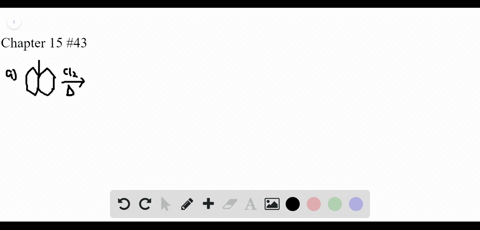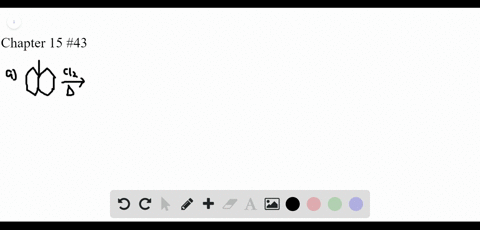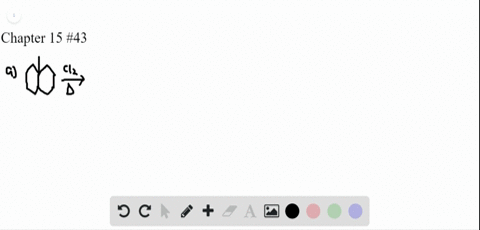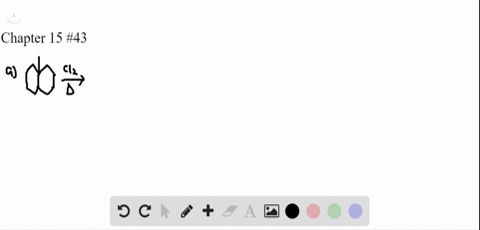
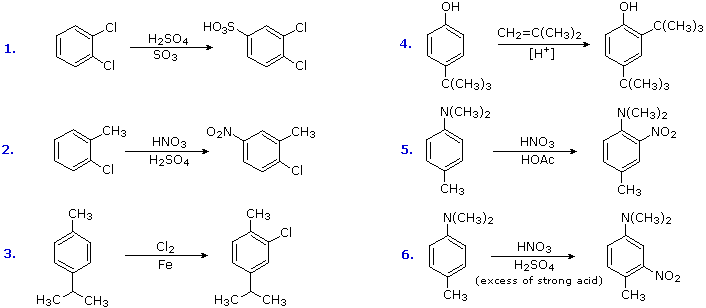
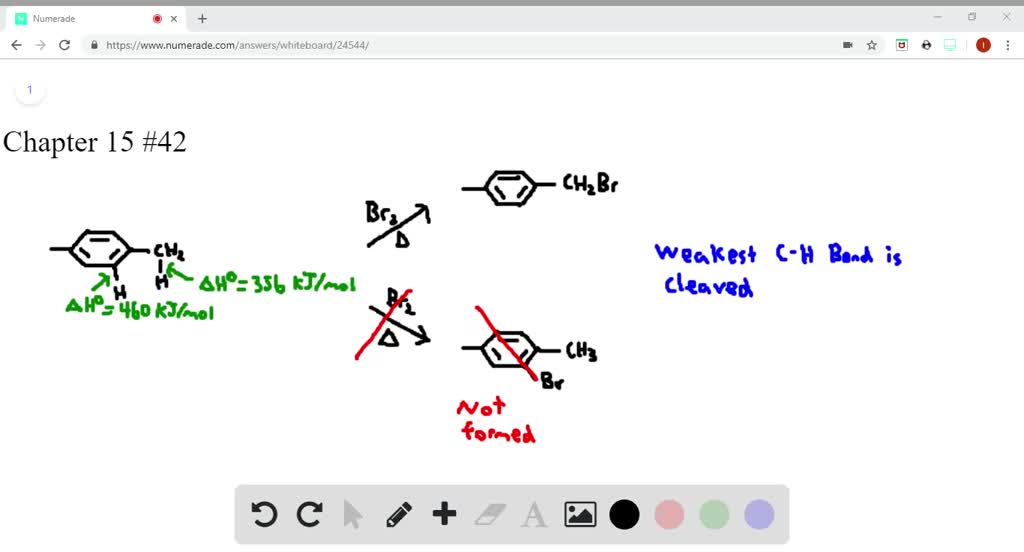
P-xylene bromination-Organic Chemistry (4th Edition) Edit edition Problem 42P from Chapter 15 Explain why radical bromination of ρxylene forms C rather t Get solutions(a) Bromination of pxylene (b) Chlorination of mxylene (c) Nitration of acetophenone (d) Friedel–Crafts acylation of anisole withCH3CCI (e) Nitration of isopropyl benzene (f) Bromination of nitrobenzene (g) Sulfonation of furan (h) Bromination of pyridineQIn each of the following pairs of compounds choose which oneIn each of the following


Solved Explain Why Radical Bromination Of P Xylen
Originally Answered Why does bromination of pxylene under heat not form a chlorobenzene derivative, but the chlorine only attaches to the methyl groups?The p stands for para, indicating that the two methyl groups in p xylene occupy the diametrically opposite substituent positions 1 and 4 It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, o xylene and m xylene All have the same chemical formula C 6 H 4 (CH 3) 2Nonetheless, recent findings suggest BrCl, Br 2 O, and BrOCl are orders of magnitude more inherently reactive (relative to HOBr) toward pxylene and the herbicide dimethenamid The extent to which brominating agents other than HOBr influence bromination rates of organic compounds beyond dimethenamid and p xylene is currently unknown
(094 mole) of dry pxylene (mp 11–12°), and the flask is heated in an oil bath maintained between 140° and 160° The stirrer is started, and, when the xylene starts boiling, 700 g (224 ml, 438 moles) of dry bromine (Note 2) is gradually added through the dropping funnel at such a rate that there is never any large amount of unreactedA process for the perbromination of phenol and diphenyl ether by brominating the corresponding compound in bromine as the sole reaction medium using metal and metalcontaining catalysts at an elevated initial reaction temperature of at least about 35° C, preferably at least about 45° C, substantially enhances reaction productivity without adversely affecting product yield or quality1627 Bromination of N,Ndimethylaniline is faster because nitrogen has an unshared electron pair that can stabilize the carbocation intermediate by resonance As in the case of oxygen, the electronwithdrawing polar effect of nitrogen is pXylene has a sixproton singlet in the benzylic region Styrene, Ph —CHACH 2, has no protons in
Bromopxylene 2bromo1,4dimethylbenzene 2,5Xylyl bromide 2Bromo1,4xylene 2,5Dimethylphenyl bromide 1,4Dimethyl2bromobenzene XYLYL BROMIDE pXylene, 2bromoMFCD NSC 8051 ATTERCOPCHM AT 2Bromopxylene, 97% NSC8051 2bromo pxylene MonobromopXylene 2bromorhoxylene EINECSVisible light induced bromination of pxylene 6 afforded mainly a product preferentially brominated at only one of the methyl groups (7a/7b = 65) This is in contrast to the reaction with bromine in water, where the reactivity was the opposite (7a/7b = 02) 14 Mesitylene 8, as well as 4methylanisole 10 and 4methylacetanilide 12 reactedA product study of pxylene oxidation by the CoBrPy catalytic system has been investigated under anaerobic conditions at 1 °CIt was shown that in brominefree systems the direct oxidation of pxylene by Co(III) ions proceeds, while the distribution and yield of reaction products are only slightly influenced by the type of solventDuring the simultaneous oxidation of pxylene and ptoluic


Organic Problems


Organic Syntheses Procedure
Q1622 If toluene is treated with D 2 SO 4 all the hydrogen's are replaced with deuterium Explain (might need to draw mechanism)J Electroanal Chem, 68 (1976) 123 Elsevier Sequoia SA, Lausanne Printed in The Netherlands Short communication ANODIC BROMINATION OF BENZENE AND NAPHTHALENE IN ACETIC ACID G CASALBORE, M MASTRAGOSTINO and S VALCHER Laboratorio di Polarografia ed Elettrochimica Preparativa del CNR, Corso Stati Uniti, Padua (Italy) and Centro di Studio di Elettrochimica Teorica eThe freeradical bromination or chlorination of aromatic side chains is well known 10 The method is suitable for activated and deactivated aromatics, and the halogenated product is converted to the desired oxidation product in a subsequent step It is probably best known for use in the production of terephthalic acid from pxylene 19 In


Www Jcsp Org Pk Articleupload 3387 1 Ce Pdf


Http Employees Oneonta Edu Knauerbr 226hw Ans 226hw5ans Pdf
We report a green and convenient protocol to prepare 4,7,12,15tetrachloro22paracyclophane, the precursor of parylene D, from 2,5dichloropxyleneIn the first bromination step, with H 2 O 2 –HBr as a bromide source, this procedure becomes organicwastefree and organicsolventfree and can appropriately replace the existing bromination methods The Winberg elimination–dimerizationThe 100% selective oxygenation of pxylene to ptolualdehyde is initiated by photoinduced electron transfer from pxylene to the singlet excited state of 10methyl9phenylacridinium ion under visible light irradiation, yielding ptolualdehyde exclusively as the final oxygenated product The reason for the high selectivity in the photocatalytic oxygenation of pxylene is discussed on the basis(Bromination) Please Include Meta, Ortho, And Para Positions For Each Product 1 Salicylic Acid > 2 Toluene > 3 Pxylene > This problem has been solved!



Answered Br2 Br R Hulene Br Not Formed Bartleby



16 E Chemistry Of Benzene Electrophilic Aromatic Substitution Exercises Chemistry Libretexts
Draw resonance structures of the intermediate carbocations in the bromination of naphthalene, and account for the fact that naphthalene undergoes electrophilic substitution at C1 rather than C2 Check back soon!In each case, how many products would be expected for the bromination of pxylene, oxylene, and mxylene?Draw resonance structures of the intermediate carbocations in the bromination of naphthalene, and account for the fact that naphthalene undergoes electrophilic substitution at C1 rather than C2 Check back soon!



Screening Of Reaction Conditions For O Bromination A B Download Table


Solved Explain Why Radical Bromination Of P Xylen
See the answer What are all possible products for these compounds when using the Br2 and acetic acid?(a) Bromination of pxylene (b) Chlorination of mxylene 9 (c) Nitration of acetophenone (d) Friedel–Crafts acylation of anisole with acetyl chloride (e) Nitration of isopropylbenzene (f) Bromination of nitrobenzene(a) Bromination of pxylene (b) Chlorination of mxylene (c) Nitration of acetophenone (d) Friedel–Crafts acylation of anisole withCH3CCI (e) Nitration of isopropyl benzene (f) Bromination of nitrobenzene (g) Sulfonation of furan (h) Bromination of pyridineQIn each of the following pairs of compounds choose which oneIn each of the following



Pdf Environmentally Benign Electrophilic And Radical Bromination On Water H2o2 Hbr System Versus N Bromosuccinimide



Figure 2 From Reactivities Of Hypochlorous And Hypobromous Acid Chlorine Monoxide Hypobromous Acidium Ion Chlorine Bromine And Bromine Chloride In Electrophilic Aromatic Substitution Reactions With P Xylene In Water Semantic Scholar
Bromination of methoxybenzene (anisole) is very fast and gives mainly the parabromo isomer, accompanied by 10% of the orthoisomer and only a trace of the metaisomer Bromination of nitrobenzene requires strong heating and produces the metabromo isomer as the chief product(Bromination) Please include meta, ortho, and para positions for eachThis is because at high temperatures free radical mechanism takes place and as a result chlorine gets attached to the methyl groups


1



Synthesis Of Arylbromides From Arenes And N Bromosuccinimide Nbs In Acetonitrile A Convenient Method For Aromatic Bromination
3 pXYLENE (3) The setup in 1(a) was used To a stirred suspension of 3 (245 cm 3, 0 mmol) in 50 cm 3 H 2 0, was added Br 2 (103 cm 3, 0 mmol) in one batch The mixture was irradiated with incandescent light for minutes after which time bromine color disappeared completelyPerhaps the direct bromination of pxylene would proceed smoother, the bromine should still take the same position and the following oxidation should still proceed fine just switch the steps I wonder if we could use the procedure they describe in Post (Rhodium "Many routes to 3,4,5Trimethoxybenzaldehyde", Methods Discourse)USA US1529A USA USA US A US A US A US 1529 A US1529 A US 1529A US A US A US A US A US A US A Authority US United States Prior art keywords bromination grams bromide bromine benzene Prior art date Legal status (The legal status is an assumption and is not a legal conclusion



Which Of The Following Structures Most Closely Represents As Intermediate In The Electrophilic Bromination Of Para Xylene


Solved Could You Kindly Solve This And Explain Why The In Chegg Com
Xylene (from Greek ξύλο, xylo, "wood"), xylol or dimethylbenzene is any one of three isomers of dimethylbenzene, or a combination thereof With the formula (CH 3) 2 C 6 H 4, each of the three compounds has a central benzene ring with two methyl groups attached at substituentsThey are all colorless, flammable liquids, some of which are of great industrial value11 Comparison of rho values with method of benzylic bromination at 70° 32 12 Effect of temperature change on the relative rate of photoinitiated bromination by BrCC13 for pmethoxytoluene vs pxylene 38 13 Effect of BrCC13 concentration on the relative rate of photoinitiated bromination by BrCC13 for pmethoxytoluene vs pxylene at 70What is claimed is 1 Selective bromination process for 2,5dibromopxylene which comprises contacting pxylene with bromine at a temperature of about °C to 40°C in the presence of from about 025 to about 50 mole percent based on the bromine of a hydrated iron containing catalyst having from about two to about six molecules of water per atom of iron present in the catalyst



Bromination Of Aromatic Compounds By Residual Bromide In Sodium Chloride Matrix Modifier Salt During Heated Headspace Gc Ms Analysis Semantic Scholar
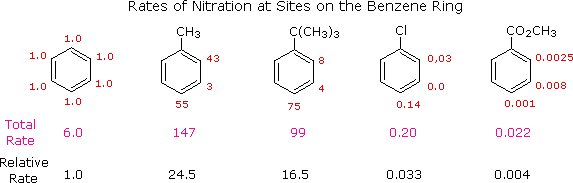


Aromatic Reactivity
Q1622 If toluene is treated with D 2 SO 4 all the hydrogen's are replaced with deuterium Explain (might need to draw mechanism) Solutions S1621 S1622 The deuterium is added to the ring When the ring "rearomatizes" theThe stirrer is started, and, when the xylene starts boiling, 700 g (224 ml, 438 moles) of dry bromine (Note 2) is gradually added through the dropping funnel at such a rate that there is never any large amount of unreacted bromine in the flask Stirring and heating are continued throughout the reaction, which requires 6–10 hoursDuring pxylene metab in perfused lungs, derivatives which became covalently bound to lung proteins were formed which suggests that pxylene metab might proceed at least partially through reactive intermediate(s) causing destruction of pulmonary cytochrome P450



Solved 10 Which Of The Following Compounds Will Not Unde Chegg Com



What Product Are Formed When The Following Compound Is Treated With Br2 In The Presence Of Febr3
Liquid phase bromination of tetrafluoropxylene Tetrafluoropxylene (361 g 98% pure pisomer) was heated to reflux in flask 11 and bromine (105 ml) added slowly through the sidearm 16 over 3 hours, after which the stillhead temperature was 158°CBeilstein Journal of Organic Chemistry 12(1)USA USA USA USA US A US A US A US A US A US A US A US A US A US A US A US A Authority US United States Prior art keywords xylene chlorination ring chlorinated reaction Prior art date Legal status (The legal status is an assumption and is not a legal conclusion



Pdf Carbon Tetrabromide A New Brominating Agent For Alkanes And Arylalkanes Vladimir Smirnov Academia Edu



16 E Chemistry Of Benzene Electrophilic Aromatic Substitution Exercises Chemistry Libretexts
Conversion of pxylene (8) by PV bromination occurred in 70 % and besides benzyl bromide 9, α,αЈdibromopxylene (10) also formed Mesitylene (11)The mechanism of electrochemical bromination of aromatic compounds in anhydrous acetic acid has been investigated Bromine ion gives two waves in anhydrous acetic acid, by addition of p xylene or toluene At the first wave Br 2 is formed which gives rise to an oxidizable complex with the aromatic substrate6 Propylation of pxylene 7 Bromination of toluene Overview – This is a 3week lab Week 1 – run reaction obtain crude product Week 2 – analyze crude samples and recrystallize Week 3 – analyze pure samples Reaction Procedures General Run the reaction using the apparatus indicated in each procedure


Organic Syntheses Procedure



Nitrate Mediated Oxidation Of P Xylene By Emulsion Electrolysis Sciencedirect
Questions Q1621 In each case, how many products would be expected for the bromination of pxylene, oxylene, and mxylene?PXYLENE (3) The setup in 1(a) was used To a stirred suspension of 3 (245 cm 3, 0 mmol) in 50 cm 3 H 2 0, was added Br 2 (103 cm 3, 0 mmol) in one batch The mixture was irradiated with incandescent light for minutes after which time bromine color FreeRadical Bromination of Selected Organic Compounds in Water11 Comparison of rho values with method of benzylic bromination at 70° 32 12 Effect of temperature change on the relative rate of photoinitiated bromination by BrCC13 for pmethoxytoluene vs pxylene 38 13 Effect of BrCC13 concentration on the relative rate of photoinitiated bromination by BrCC13 for pmethoxytoluene vs pxylene at 70
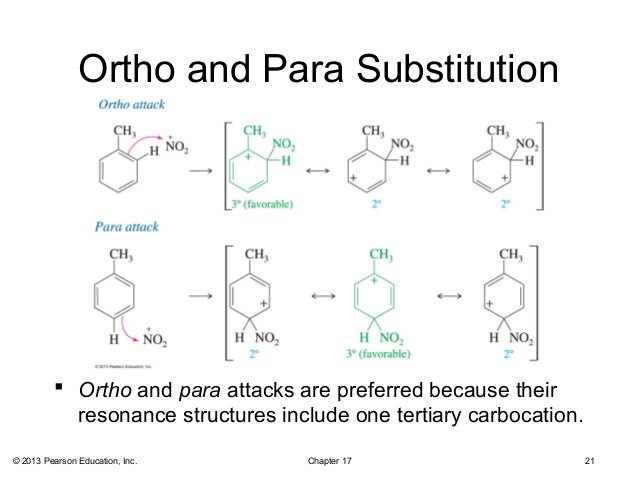


17 Lecture


Crab Rutgers Edu Alroche Ch17 Pdf
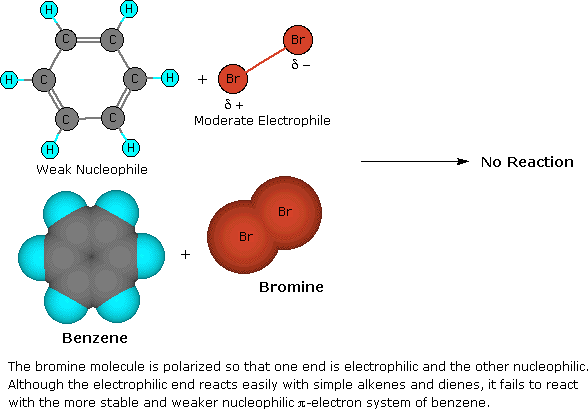
Addition reactions In the presence of ultraviolet light (but without a catalyst present), hot benzene will also undergo an addition reaction with chlorine or bromine The ring delocalisation is permanently broken and a chlorine or bromine atom adds on to each carbon atomLiquid phase bromination of tetrafluoropxylene Tetrafluoropxylene (361 g 98% pure pisomer) was heated to reflux in flask 11 and bromine (105 ml) added slowly through the sidearm 16 over 3 hours, after which the stillhead temperature was 158°CWhen you react toluene with Br2 in the presence of FeBr3 catalyst, you will get a mixture of 2bromotoluene and 4bromotoluene The CH3 group is a ortho and para directing activator for electrophilic substitution on the benzene ring


Crab Rutgers Edu Alroche Ch17 Pdf
.png?revision=1)


16 E Chemistry Of Benzene Electrophilic Aromatic Substitution Exercises Chemistry Libretexts
There are three products that might form on bromination of toluene Draw and name them pxylene (a) mdinitrobenzene, (b) o and pbromonitrobenzene, (c) o and pnitrotoluene, (d) mnitrobenzoic acid, (e) 1,4dimethyl2nitrobenzene 15 Draw resonance structures of the three possible carbocation intermediates to show how aLoose Leaf for Organic Chemistry (5th Edition) Edit edition Problem 40P from Chapter 15 Explain why radical bromination of pxylene forms C rather t Get solutionsIn the first bromination step, 1(bromomethyl)2,5dichloro4methylbenzene was synthesized with high yield and selectivity from 2,5dichloropxylene by using a H 2 O 2 –HBr couple in water The use of H 2 O 2 –HBr as a bromide source made this procedure organicwastefree, organicsolventfree and an appropriate replacement of the
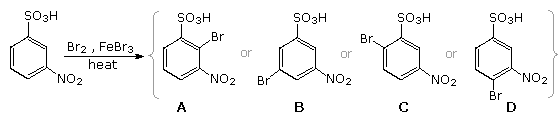


Organic Problems



Media Portfolio
A new protocol for the synthesis of 4,7,12,15tetrachloro22paracyclophane November 16;
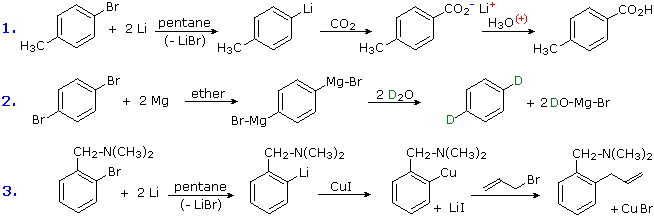


Aromatic Reactivity
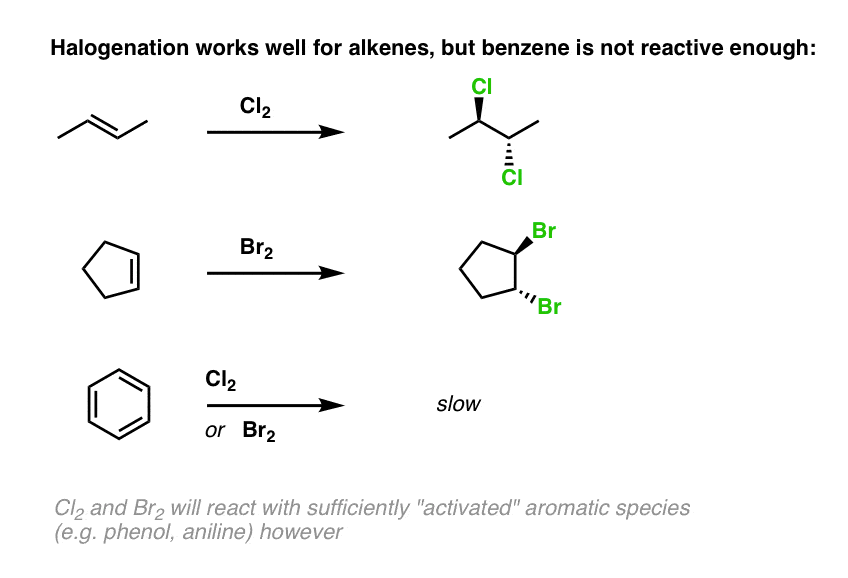


Electrophilic Aromatic Substitutions Chlorination And Bromination
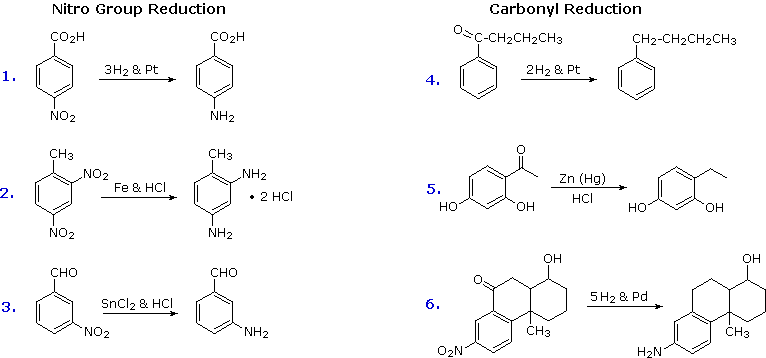


Aromatic Reactivity


Solved Arrange The Following Compounds In Order Of Decrea Chegg Com



Woa1 Preparation Of 4 Bromo O Xylene Google Patents


Crab Rutgers Edu Alroche Ch17 Pdf



Meta Xylene An Overview Sciencedirect Topics
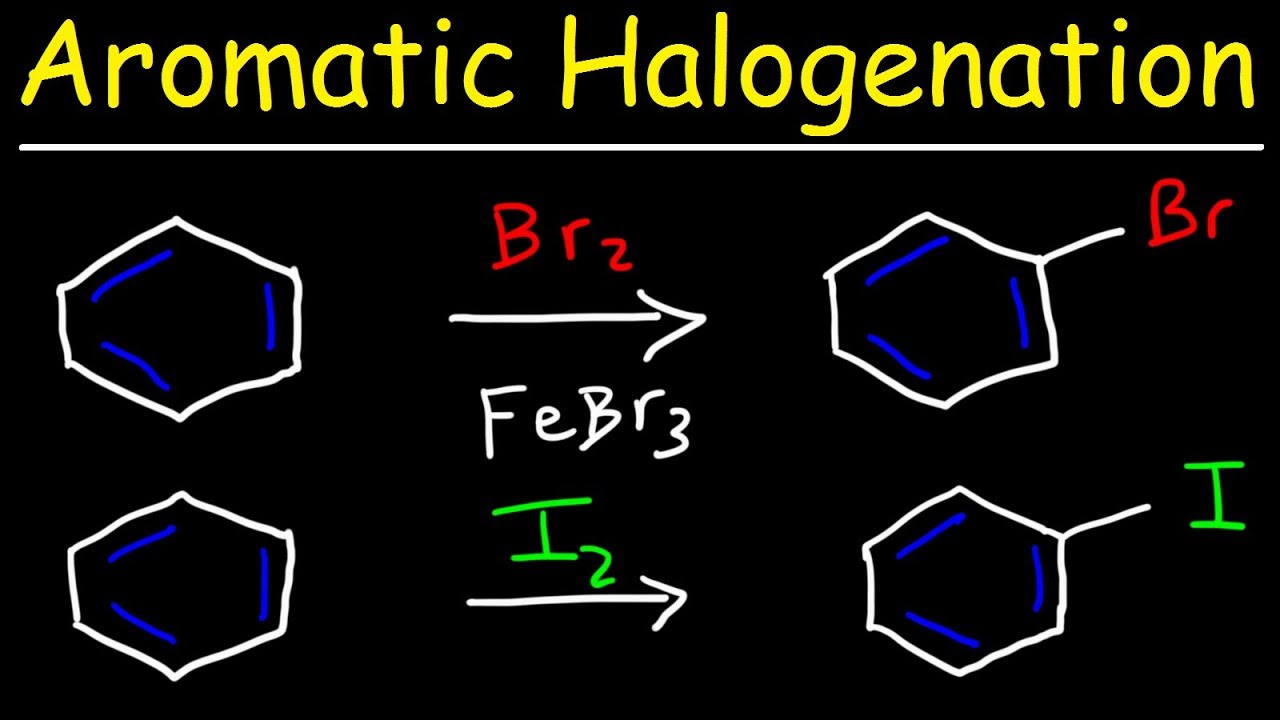


Aromatic Halogenation Mechanism Chlorination Iodination Bromination Of Benzene Youtube



Pdf Environmentally Benign Electrophilic And Radical Bromination On Water H2o2 Hbr System Versus N Bromosuccinimide
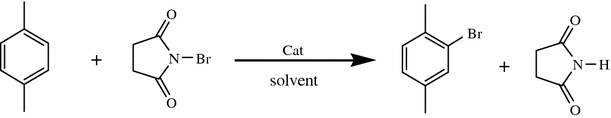


Table 1 Silver Catalyzed Bromination Of Aromatics With N Bromosuccinimide Springerlink



Xylene Tree Of Knowledge Wiki Fandom



16 E Chemistry Of Benzene Electrophilic Aromatic Substitution Exercises Chemistry Libretexts


Chapter 16
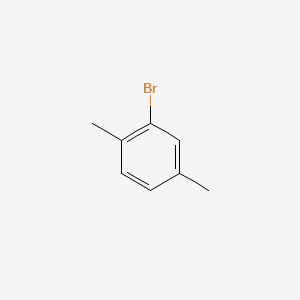


2 Bromo P Xylene C8h9br Pubchem



What Product Are Formed When The Following Compound Is Treated With Br2 In The Presence Of Febr3


Q Tbn And9gctbcss6mhjmkhejlitoccmkr4dvhn0wzim9w0lj1ksqgn0kjwje Usqp Cau
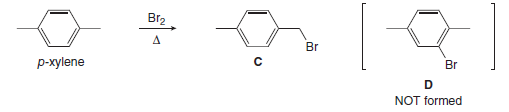


Solved Explain Why Radical Bromination Of P Xylene Forms C Rat Chegg Com
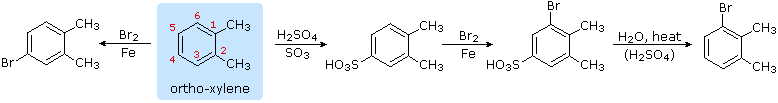


Aromatic Reactivity



Chapter 13 Problems Pdf Chapter 13 Problem Set 1 Write The Structure Of The Organic Product In Each Of The Following Reactions If Electrophilic Course Hero



Today Conclusion Of Friedel Crafts Gc Ppt Video Online Download



Solved Chapter 18 Reactions Of Substituted Benzenes The Chegg Com


2



Electrophilic Aromatic Substitution



Solved Explain Why Radical Bromination Of P Xylen


Crab Rutgers Edu Alroche Ch17 Pdf



Chem 12b Exam 1 Flashcards Quizlet



Bromination From The Macroscopic Level To The Tracer Radiochemical Level 76 Br Radiolabeling Of Aromatic Compounds Via Electrophilic Substitution Abstract Europe Pmc



Pdf Environmentally Benign Electrophilic And Radical Bromination On Water H2o2 Hbr System Versus N Bromosuccinimide
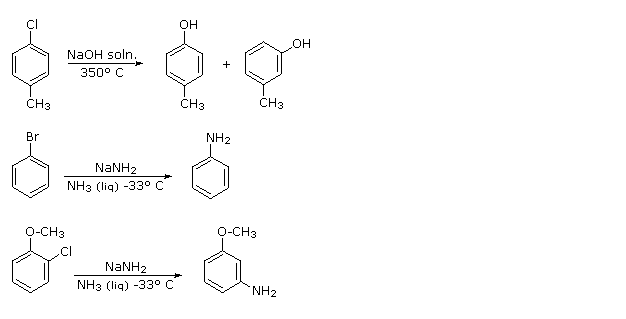


Aromatic Reactivity


Q Tbn And9gctk6ue5yjkbxypkvtetue9ijc44tswsq3t3qnx7ke9k Itf6v Usqp Cau
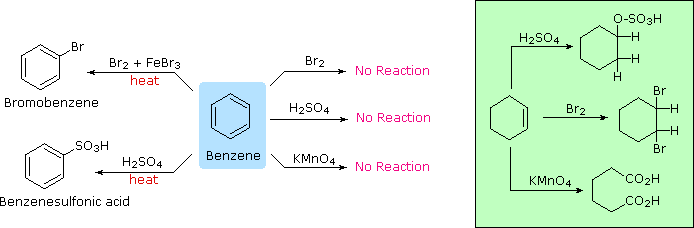


Aromatic Reactivity


Q Tbn And9gctwlk0f4obady4euujvs65nrkcaxhlruykcu3 Ng Av8axb5f3x Usqp Cau
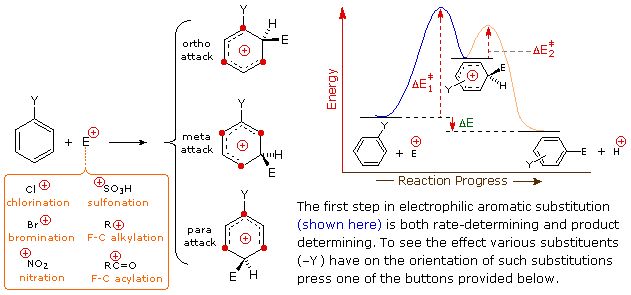


Aromatic Reactivity



Media Portfolio


Solved A Draw A Detailed Mechanism For The Reaction Of Ethyl Chegg Com


Aromatic Reactivity
.png?revision=1)


16 E Chemistry Of Benzene Electrophilic Aromatic Substitution Exercises Chemistry Libretexts


Crab Rutgers Edu Alroche Ch17 Pdf



Epa1 Bromination Of Ortho Xylene Google Patents


Organic Problems
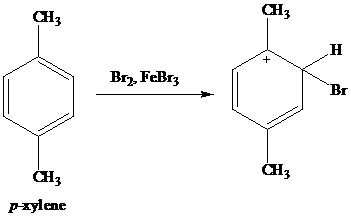


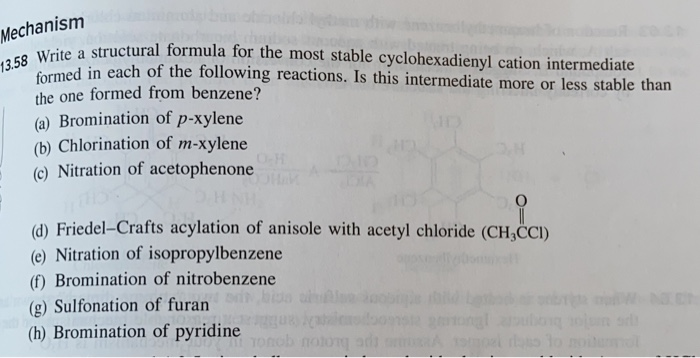
Solved Write A Structural Formula For The Most Stable Cyclohex Chegg Com


Aromatic Reactivity
.jpg?revision=1&size=bestfit&width=482&height=283)


16 2 Other Aromatic Substitutions Chemistry Libretexts


Aromatic Reactivity


Aromatic Reactivity


2


Solved Explain Why Radical Bromination Of P Xylen
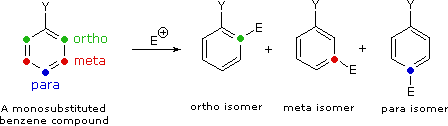


Aromatic Reactivity


Crab Rutgers Edu Alroche Ch17 Pdf



Solved 2 What Effect On Reactivity Toward The Electrophi Chegg Com
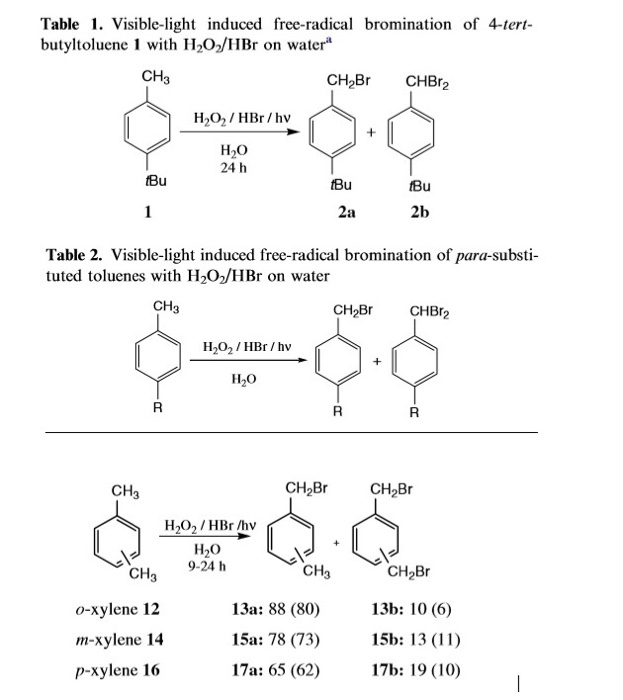


Solved I Want Mechanisms For This Reaction Free Radical Chegg Com



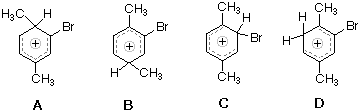
Which Of The Following Structures Most Closely Represents As Intermediate In The Electrophilic Bromination Of Para Xylene
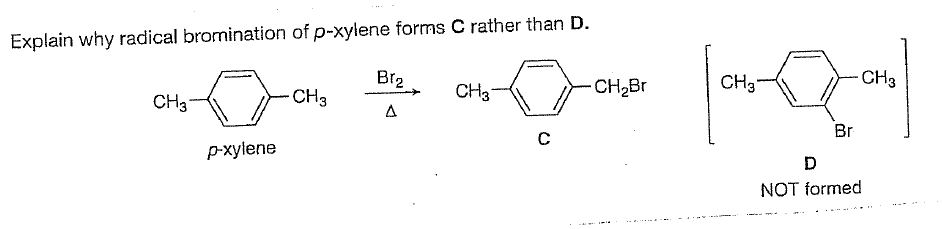


Explain Why Radical Bromination Of P Xylene Forms Chegg Com
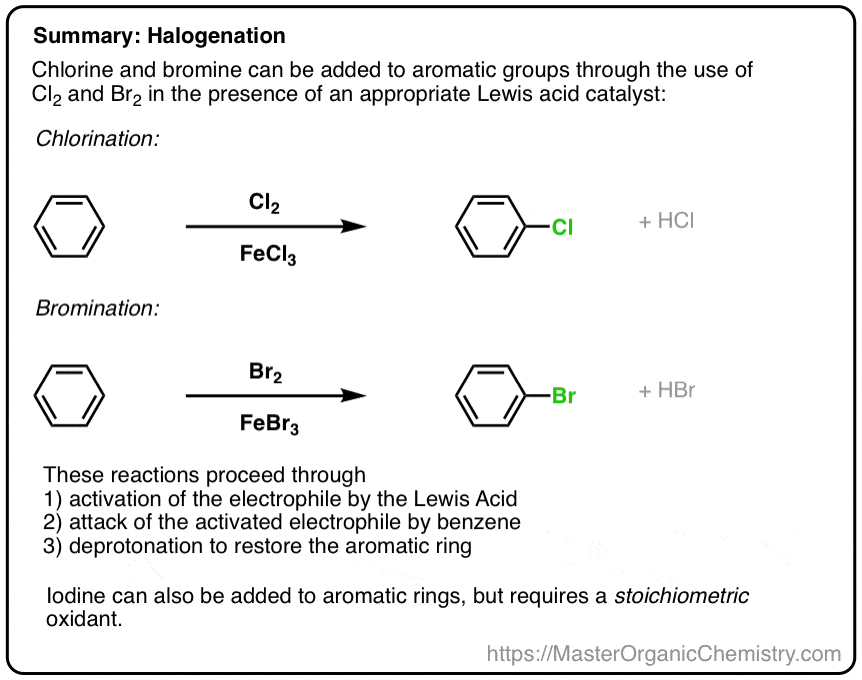


Electrophilic Aromatic Substitutions Chlorination And Bromination


2



16 E Chemistry Of Benzene Electrophilic Aromatic Substitution Exercises Chemistry Libretexts
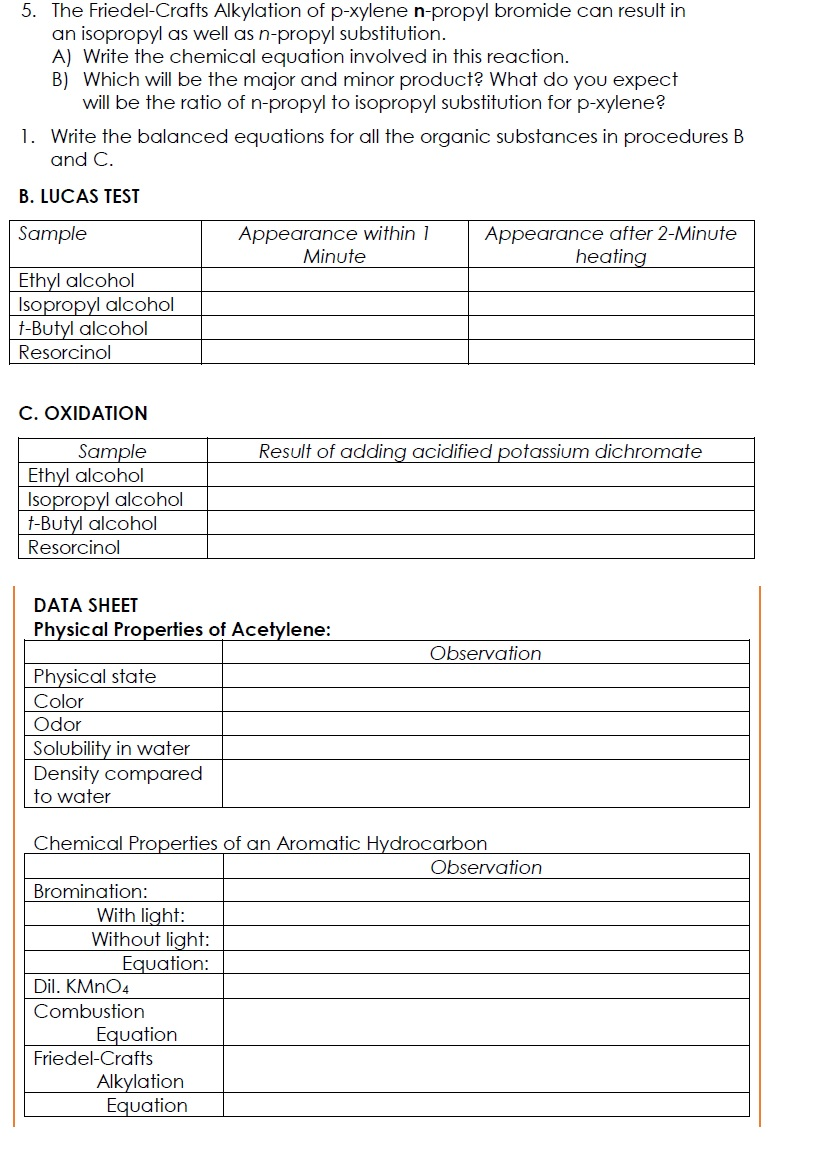


Solved 5 The Friedel Crafts Alkylation Of P Xylene N Pro Chegg Com



16 E Chemistry Of Benzene Electrophilic Aromatic Substitution Exercises Chemistry Libretexts


What Is The Reaction To Make Meta Xylene From Benzene It Is Possible To Make Para And Ortho By Direct Alkylation But How Do I Create Meta Xylene Quora



Bromination Of Aralkyl Compounds Using Potassium Bromate And Potassium Bromide In Acidic Medium By Transtellar Publications Issuu


2



Gold Mediated C H Bond Functionalisation Chemical Society Reviews Rsc Publishing Doi 10 1039 C0csa



Which Of The Following Structures Most Closely Represents As Intermediate In The Electrophilic Bromination Of Para Xylene



1 2 Dibromo 4 5 Dimethylbenzene 97 48 7 Sigma Aldrich



Woa1 Preparation Of 4 Bromo O Xylene Google Patents



Solved Explain Why Radical Bromination Of P Xylen



What Product Are Formed When The Following Compound Is Treated With Br2 In The Presence Of Febr3



Solved Questions 1 As With Nitration Bromination Requi Chegg Com



Desulfonation An Overview Sciencedirect Topics


2



Bromination Of Aromatic Compounds By Residual Bromide In Sodium Chloride Matrix Modifier Salt During Heated Headspace Gc Ms Analysis Semantic Scholar



Figure 1 From Reactivities Of Hypochlorous And Hypobromous Acid Chlorine Monoxide Hypobromous Acidium Ion Chlorine Bromine And Bromine Chloride In Electrophilic Aromatic Substitution Reactions With P Xylene In Water Semantic Scholar



Synthesis Of Arylbromides From Arenes And N Bromosuccinimide Nbs In Acetonitrile A Convenient Method For Aromatic Bromination



Table 11 From Reactivities Of Hypochlorous And Hypobromous Acid Chlorine Monoxide Hypobromous Acidium Ion Chlorine Bromine And Bromine Chloride In Electrophilic Aromatic Substitution Reactions With P Xylene In Water Semantic Scholar
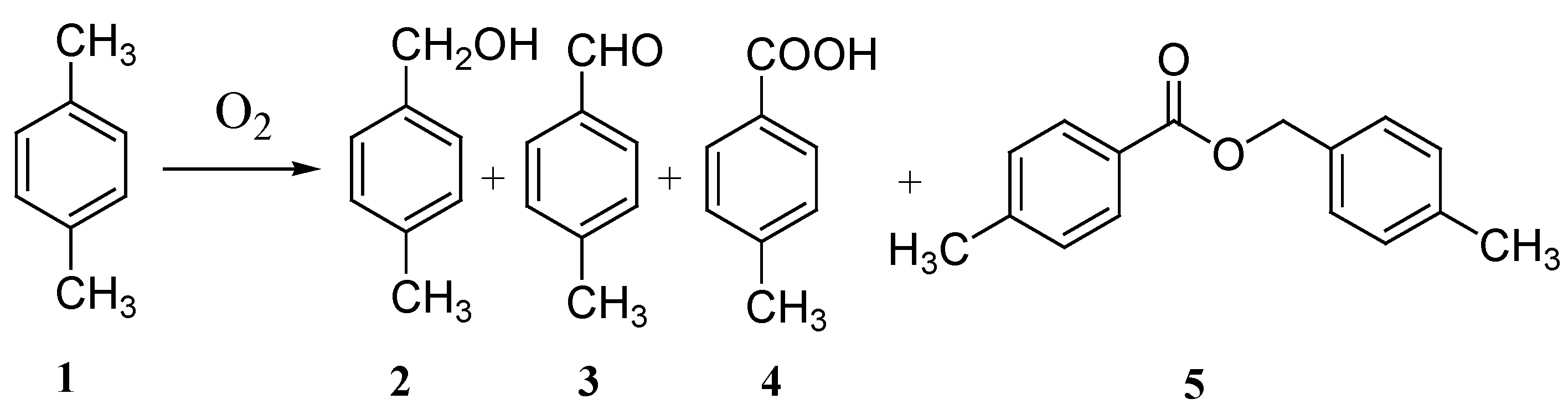


Molecules Free Full Text 4 N N Dimethylaminopyridine Promoted Selective Oxidation Of Methyl Aromatics With Molecular Oxygen Html
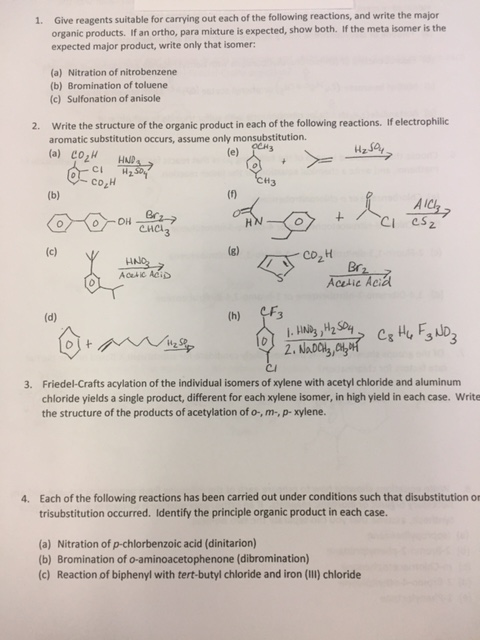


Solved 1 Give Reagents Suitable For Carrying Out Each Of Chegg Com


コメント
コメントを投稿